The Pfizer-BioNTech vaccine has been shown to be effective in preventing COVID-19 hospitalizations. While the level of protection varies across studies, it is generally accepted that the vaccine provides a significant reduction in the risk of hospitalization. The original Pfizer vaccine, introduced in December 2020, has since been updated multiple times to target different variants of the virus, including Omicron. While the vaccine does not offer complete protection against COVID-19 hospitalization, it is still recommended, especially for high-risk groups, as it can reduce the severity of the disease and help manage the ongoing pandemic.
What You'll Learn
- Pfizer vaccine's effectiveness against SARS-CoV-2 infections declines over time
- Pfizer's two doses are 93% effective against the Delta variant
- Pfizer's two doses are 97% effective against non-Delta variants
- Pfizer's two doses are 90% effective against Covid-19 hospitalisations
- Pfizer's two doses are safe for children aged 6 months to 4 years

Pfizer vaccine's effectiveness against SARS-CoV-2 infections declines over time
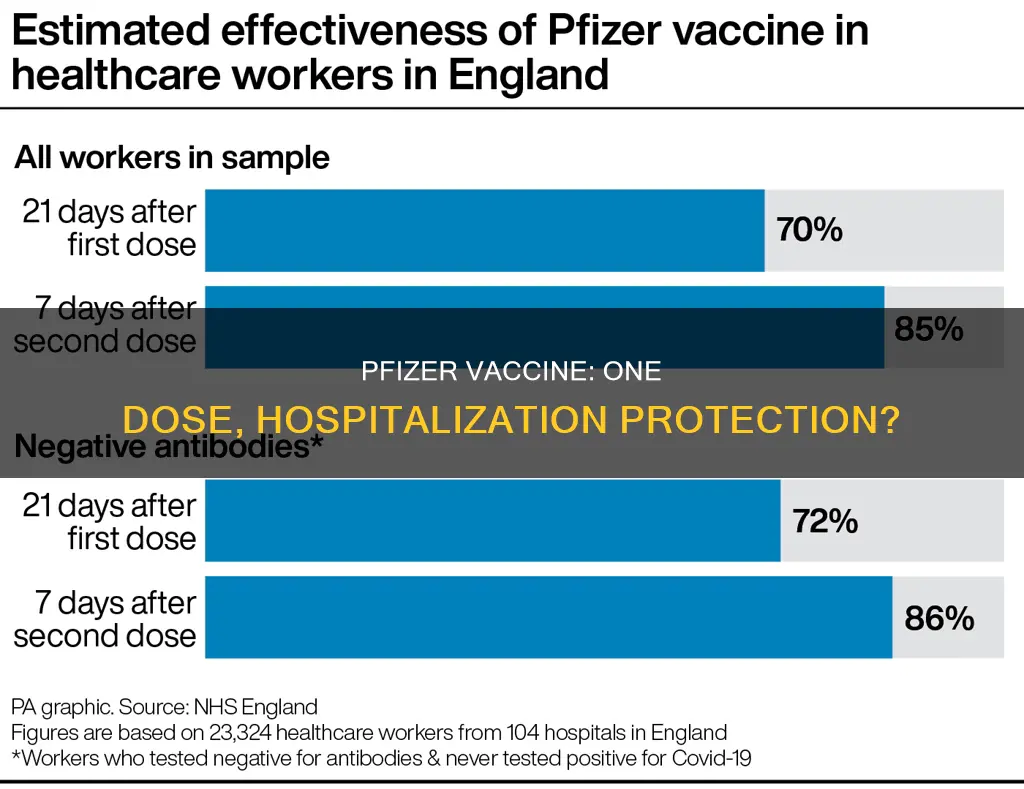
While the Pfizer vaccine is highly effective at preventing COVID-19 hospitalizations, its effectiveness against SARS-CoV-2 infections declines over time. A study by Kaiser Permanente Southern California and Pfizer found that the vaccine's effectiveness against all SARS-CoV-2 infections decreased over a six-month period. Within the first month after receiving two vaccine doses, the effectiveness was 88%, but it dropped to 47% after six months.
The study also examined the vaccine's effectiveness against the delta variant. One month after the second dose, it was 93% effective, but this decreased to 53% after four to five months. Similarly, the vaccine's effectiveness against non-delta variants declined from 97% in the first month to 67% at four to five months.
Another study by the UNC Gillings School of Global Public Health found that COVID-19 booster shots targeting the XBB.1.5 subvariant were highly effective at preventing hospitalization. The vaccines showed 66.8% effectiveness in preventing hospitalization one month after receiving the booster. However, similar to the previous study, the effectiveness of the boosters also waned over time, with a decrease to 57.1% after 10 weeks.
These findings highlight the importance of monitoring vaccine effectiveness and considering booster shots to maintain protection against COVID-19 infections and hospitalizations. The development of updated vaccines that target specific strains, such as the annual deployment of new vaccines by the FDA, is also crucial to address the evolving nature of the SARS-CoV-2 virus and its variants.
While the Pfizer vaccine's effectiveness against infections may wane over time, it is still expected to provide protection against severe disease, hospitalization, and death from COVID-19. The goal of the vaccines is to prevent severe illness, and they have been updated annually since 2022 to address emerging variants.
MRSA: A Hospital Hazard and Its Challenges
You may want to see also

Pfizer's two doses are 93% effective against the Delta variant
According to a study published in The Lancet, two doses of the Pfizer-BioNTech vaccine are 93% effective in preventing Covid-19 hospitalizations due to the Delta variant. The study, a joint effort by Kaiser Permanente Southern California and Pfizer, underscores the importance of improving global Covid-19 vaccination rates and monitoring vaccine effectiveness to determine which populations should receive booster shots.
While the Pfizer vaccine has proven highly effective against the Delta variant, it is important to note that vaccine effectiveness wanes over time. The study found that the vaccine's effectiveness against Delta variant infections was 93% one month after receiving two doses but fell to 53% after four months. This decline highlights the need for ongoing monitoring of vaccine effectiveness and the potential requirement for booster shots to maintain protection against Covid-19 variants.
The Pfizer-BioNTech vaccine has been a critical tool in the fight against Covid-19. In December 2020, Phase 3 clinical data for the original Pfizer vaccine showed 95% efficacy in preventing symptomatic Covid-19. The vaccine was the first to receive FDA Emergency Use Authorization, and it played a pivotal role in the initial stages of the vaccination rollout.
As the SARS-CoV-2 virus continued to mutate and evolve, the Pfizer vaccine underwent updates to target new variants. The original mRNA vaccines from Pfizer and Moderna, introduced in December 2020, protected against the initial SARS-CoV-2 virus. However, as new variants emerged, such as the Omicron strain and its subvariants, the vaccines were updated accordingly. This continuous adaptation of the vaccine technology ensures that we stay ahead of the virus and maintain protection against hospitalization and severe disease.
In summary, while two doses of the Pfizer vaccine are highly effective against the Delta variant, it is important to stay up-to-date with the latest vaccine recommendations. The ongoing evolution of the SARS-CoV-2 virus and the emergence of new variants require us to remain vigilant and proactive in our vaccination efforts to prevent severe disease, hospitalization, and death.
Edna Adan's Journey: Building a Hospital
You may want to see also

Pfizer's two doses are 97% effective against non-Delta variants
While it is true that Pfizer's two doses are 97% effective against non-Delta variants, the effectiveness of the vaccine against all SARS-CoV-2 infections declined over time. According to a study, the effectiveness of two doses of the Pfizer vaccine against non-Delta variants was 97% after one month and declined to 67% after four months.
Pfizer's Covid-19 vaccine has proven to be significantly less effective at preventing infection with the Delta variant compared to previous strains of coronavirus. However, it is still highly effective at preventing serious illness and hospitalization. An early study by Israel's health ministry reported that the Pfizer vaccine was 64% effective at preventing symptomatic Covid-19, much lower than previous estimates of nearly 90%. Nonetheless, the vaccine remained 93% effective at preventing hospitalization, although this was slightly lower than earlier studies.
The original Covid mRNA vaccines from Pfizer and Moderna, first introduced in December 2020, protected against the original SARS-CoV-2 virus. Since then, these vaccines have been updated multiple times to target different iterations of the Omicron strain. In 2022, bivalent vaccines were introduced to target both the original virus and the Omicron BA.4 and BA.5 variants. In 2023, a monovalent shot was developed to target the XBB lineage of the Omicron variant. The 2024-2025 updated vaccines are designed to protect against the currently circulating strains, including the Omicron KP.2 variant.
It is important to note that vaccine protection decreases over time, and the SARS-CoV-2 virus continues to mutate and evolve. Therefore, it is recommended to stay up-to-date with the latest Covid-19 vaccines to ensure optimal protection against severe illness, hospitalization, and death. The 2024-2025 vaccines are expected to provide effective protection against predominant strains and emerging variants.
Grey Sloan Memorial Hospital: Fact or Fiction?
You may want to see also

Pfizer's two doses are 90% effective against Covid-19 hospitalisations
While it is true that two doses of the Pfizer vaccine are 90% effective at preventing hospitalisation from COVID-19, this effectiveness wanes over time. A study published in The Lancet in 2021 found that the vaccine's effectiveness against all SARS-CoV-2 infections declined from 88% within one month after receiving two vaccine doses to 47% after six months. However, the effectiveness against hospitalisations remained at 90% overall for all variants, including Delta.
The study underscores the importance of improving COVID-19 vaccination rates worldwide and monitoring vaccine effectiveness to determine which populations should receive booster shots. According to the researchers, "vaccines are a critical tool for controlling the pandemic and remain highly effective in preventing severe disease and hospitalisation".
While the two doses of the Pfizer vaccine provide strong protection against hospitalisation, additional studies have shown that a third dose offers even greater protection. Research published in the Journal of Infectious Diseases in 2024 found that a third dose of the Pfizer vaccine provided significant protection against hospitalisation and death compared to two doses, especially within the first four months after vaccination.
It is important to note that the effectiveness of COVID-19 vaccines can vary depending on the circulating variants. The original mRNA vaccines from Pfizer and Moderna, introduced in December 2020, have been updated multiple times to target different variants, including Omicron and its subvariants. As the SARS-CoV-2 virus continues to mutate and new variants emerge, it is crucial to stay up to date with the recommended vaccines to ensure optimal protection against severe disease, hospitalisation, and death.
Error Reporting: Saving Hospitals Money
You may want to see also

Pfizer's two doses are safe for children aged 6 months to 4 years
A study published in The Lancet has shown that two doses of Pfizer-BioNTech (BNT162b2) are 90% effective in preventing COVID-19 hospitalizations across all variants. The effectiveness against all SARS-COV-2 infections declined from 88% within a month of receiving two doses to 47% after six months, indicating the need for a booster dose.
Pfizer-BioNTech has been deemed safe for children aged 6 months to 4 years. During the period of June 17, 2022, to May 7, 2023, 2,969 children aged 6 months to 5 years received a third COVID-19 vaccine dose and completed at least one health survey. Of these, 1,082 children aged 6 months to 2 years, and 823 children aged 3 to 4 years, received the Pfizer-BioNTech vaccine. Local and systemic reactions were reported for 930 (53.2%) children on the day after vaccination, with 1,119 (37.7%) showing no reactions. Local reactions were reported for 215 (19.9%) children aged 6 months to 2 years, and 304 (36.9%) aged 3 to 4 years. Systemic reactions were reported for 617 (57.0%) children aged 6 months to 2 years, and 361 (43.9%) children aged 3 to 4 years. The most common reactions were irritability or crying, injection site pain, sleepiness, and fever. Most reactions were described as mild, and serious adverse events were rare.
Pfizer-BioNTech was initially granted FDA Emergency Use Authorization (EUA) in December 2020. In June 2022, the FDA amended the EUA to include children aged 6 months to 4 years, recommending three doses of 3 μg (0.2 mL) each. Clinical trials involved 3,013 children in this age group, and most adverse events reported were mild to moderate, with no serious vaccine-related adverse events.
The CDC recommends that children aged 6 months to 4 years receive multiple doses, including at least one dose of the 2024-2025 updated vaccine.
Seattle Grace Hospital: Revival and Resilience
You may want to see also
Frequently asked questions
The Pfizer vaccine has been shown to prevent COVID-19 hospitalizations by 90% for at least six months after two doses. However, the effectiveness against SARS-CoV-2 infections declined over time, falling from 88% within a month after two doses to 47% after six months. While the vaccine helps prevent severe illness and hospitalization, it is important to stay up to date with the recommended doses and boosters to maintain protection against new variants.
The Pfizer vaccine has been found to be highly effective in preventing COVID-19 hospitalizations. Studies have shown that two doses of the vaccine can provide up to 93% effectiveness against the Delta variant hospitalizations and 97% against other variants for at least one month.
The protection provided by the Pfizer vaccine wanes over time. Effectiveness against all SARS-CoV-2 infections declined from 88% within one month to 47% after six months. Therefore, it is important to stay up to date with the recommended vaccine doses and boosters to maintain protection.
The Pfizer vaccine has undergone rigorous testing and is generally considered safe. However, as with any vaccine, there is a rare possibility of adverse events. It is always recommended to consult with a qualified healthcare professional to understand the benefits and risks of the vaccine for your specific situation.